

BENZA BLOCK TROCHE
ベンザブロックトローチ
CHARACTERISTICS
- This medicine is a troche containing the antiseptic ingredient for the oral cavity, cetylpyridinium chloride hydrate, and controls swollen throat and sore throat due to throat inflammation.
- This medicine contains dextromethorphan phenolphthalinate, an antitussive ingredient that acts on the cough center and controls severe cough.
- This medicine is a green donut-shaped troche. It is the first troche containing powdered green tea (flavoring agent).
- This medicine can be taken by all family members, including children aged 5 years or over.
INDICATIONS
Cough, phlegm.
Swollen throat, sore throat, rough throat, throat discomfort and hoarse voice due to throat inflammation.
DOSAGE AND DIRECTIONS
Take the following amount of medicine by holding the troche in the mouth and slowly melting it without chewing.
| Age | One dose | Daily dose | Dosing interval |
| 11 years or over | 1 troche | 6 times | 2 hours or longer |
| 8 - 10 years | 4 times | 4 hours or longer | |
| 5 - 7 years | 3 times | ||
| Under 5 years | Do not take. | ||
Precautions regarding dosage and directions
- Children are permitted to take this medicine only under the direction and supervision of a parent or other responsible adult.
- Please follow the recommended dosage and directions.
- How to take out a troche
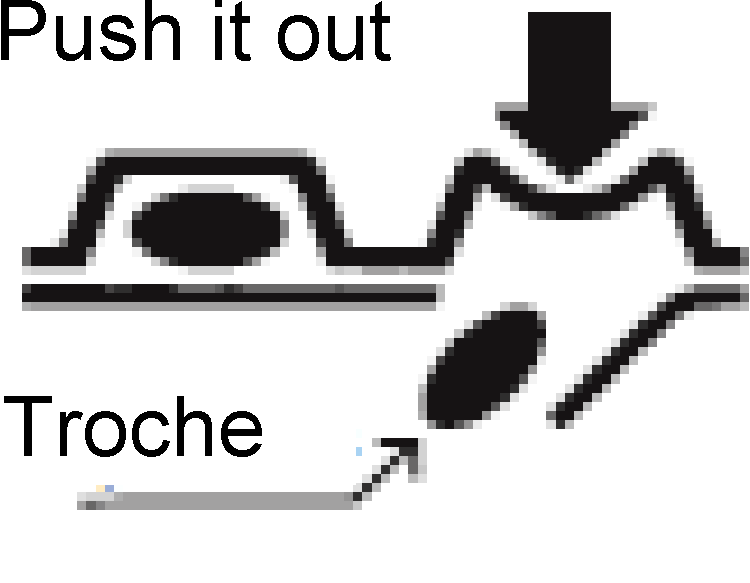
As shown in the figure, press the protruded portion of the PTP sheet with your finger to tear the aluminum foil and take out a troche. (If you swallow the PTP sheet by mistake, its sharp edges may perforate your esophagus, etc., resulting in serious complications.)
INGREDIENTS
In 6 troches (daily dose for persons aged 11 years or over)
| ACTIONS | INGREDIENTS | CONTENTS |
|---|---|---|
| Cough, phlegm, and swollen throat and sore throat due to throat inflammation |
Dextromethorphan phenolphthalinate | 60mg |
| Potassium guaiacolsulfonate | 140mg | |
| Cetylpyridinium chloride hydrate | 6mg |
Inactive ingredients:
Glucose, powdered green tea, sodium copper chlorophyllin, hydrogenated oil, magnesium stearate, mentha oil, l-menthol, dextrin, acacia, flavor, starch sodium octenylsuccinate, ethanol, vanillin, sucrose
PRECAUTIONS FOR USE
What you should NOT do
(If you do not follow the precautions listed below, your current symptoms may worsen and you may be at an increased risk of side effects.)
- The following persons should not take this medicine:
Persons who have had allergies to this medicine or ingredients of this medicine - Do not use any of the following medicines while you take this medicine:
Other cough and expectorant drugs, cold medicines, oral remedies containing an antihistamine agent (such as oral remedies for rhinitis, motion sickness drugs, medicines for allergies, and sedative hypnotics), etc., sedating drugs
Who should seek consultation
- The following persons should consult a physician, pharmacist, or registered salesperson* before taking this medicine:
- Persons under treatment by a physician
- Women who are or may be pregnant
- Persons who have had allergies to medicines or something
- Persons who have suffered from the following symptom:
High fever
- Stop taking this medicine immediately and consult a physician, pharmacist, or registered salesperson if the following symptoms appear after taking this medicine because they might be side effects of the medicine. Take this leaflet with you.
Affected area Symptoms Skin Rashes, redness, itching Digestive organs Nausea, vomiting, loss of appetite Neuropsychiatric system Dizziness Respiratory system Breathing difficulties, shortness of breath In rare cases, serious symptoms might occur. In such a case, immediately consult a physician.
Names of symptoms Symptoms Shock
(Anaphylaxis)Symptoms such as itching of the skin, urticaria, hoarseness, sneezing, itching of the throat, breathing difficulties, palpitations, and clouded consciousness will appear immediately after taking the medicine. - Stop taking this medicine and consult a physician, pharmacist or registered salesperson if symptoms do not improve after taking this medicine five to six times. Take this leaflet with you.
*Registered salesperson: person who has the license to sell OTC medicines except Category 1.
PRECAUTIONS FOR STORAGE AND HANDLING
- Store the medicine in its box in a dry, cool place away from direct sunlight.
- Keep out of the reach of children.
- To prevent improper use and loss of quality, do not transfer the medicine to another container.
- Do not take this medicine after the expiration date.
- Fill in the date when the inner pouch (aluminum pouch) was opened in the column provided on the box.
- Once the inner pouch (aluminum pouch) is opened, take this medicine within about 1 month to maintain quality.
- Do not eat the desiccant included in the inner pouch (aluminum pouch).
D1
